CircNUP50 was upregulated in DDP-resistant OC tissues/cells
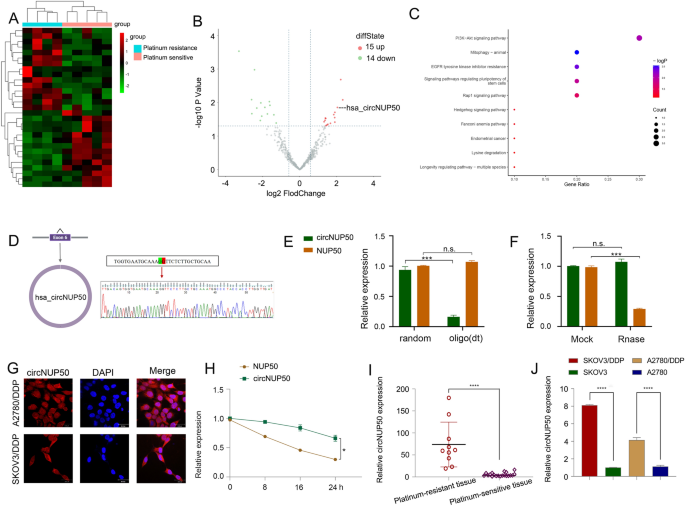
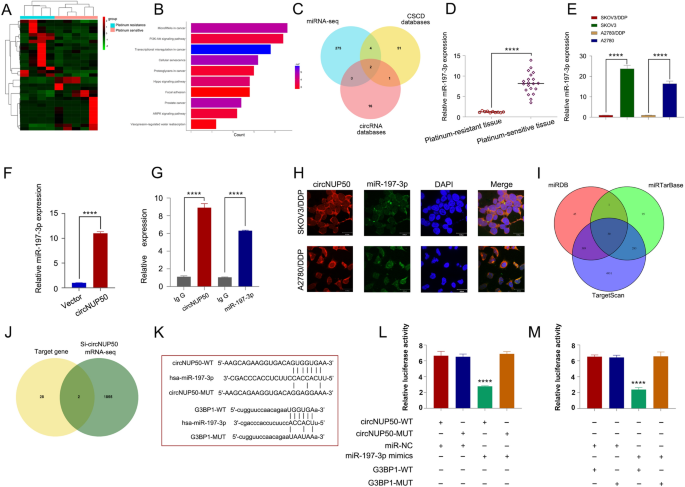
Twenty-nine circRNAs differed between platinum-resistant OC and PS tissues (Fig. 1A and B). Kyoto Encyclopedia of Genes and Genomes (KEGG) (Fig. 1C) and Gene Ontology (GO) evaluation (Further file 4: Determine S1A) confirmed that the differentially expressed circRNAs had been related to the PI3K-Akt signalling pathway, optimistic progress regulation, and optimistic autophagy regulation (Further file 2: Desk S4). PI3K/AKT pathway activation promotes OC cell progress, migration, and invasion [16,17,18]. Inducing autophagy and inactivating the PI3K/AKT/mTOR pathway inhibits the malignant behaviour of OC [19]. As well as, autophagy-related signalling pathways, notably the PI3K/AKT/mTOR pathways, are upregulated in superior most cancers levels, and the malignant phenotype of the illness reduces autophagy, resulting in tumour development [20]. Thus, the PI3K-Akt pathway and autophagy are related to tumour development in OC. Autophagy is without doubt one of the causes underlying resistance to many anti-tumour medication (together with DDP). LDLR knockdown may scale back OC tumour progress by inhibiting autophagy related to the PI3K/AKT/mTOR pathway, and LDLR promoted autophagy-mediated resistance to DDP in OCs related to the PI3K/AKT/mTOR pathway [21]. Due to this fact, the PI3K-Akt pathway and autophagy are additionally related to platinum resistance in OC. These outcomes recommend that these variations in circRNAs are carefully associated to tumour development and drug resistance. We discovered three upregulated circRNAs with a log2 fold change > 2 (P > 0.05). We then performed qRT-PCR on the three circRNAs in cells and tissues, and circNUP50 (hsa_circ_0002077, 663 bp) had vital expression variations in cells and tissues. qRT-PCR primers for circNUP50 had been ready (Further file 4: Determine S1B, and S1C). Sanger sequencing confirmed that again splicing created circNUP50 from exon 6 of the NUP50 gene (Fig. 1D). qRT-PCR was used to find out whether or not circNUP50 incorporates poly(A) tails utilizing oligo (dT) and random primers. If the goal gene was not expressed within the oligo (dT) reverse-transcribed cDNA, the RNA is round and lacks a poly(A) tail. Moreover, qRT-PCR was used to verify the round nature of circNUP50 utilizing oligo (dT) and random primers. circNUP50 expression was considerably lowered in comparison with NUP50 expression when utilizing random primers, indicating that circNUP50 lacked a poly(A) tail (Fig. 1E). RNase R can solely degrade linear RNA, not circRNA, and the RNase R remedy revealed that circNUP50 was immune to RNase R (Fig. 1F). FISH assays had been additionally carried out to find out the placement of circRNA. A fluorescent sign equivalent to circNUP50 was expressed within the nucleus and cytoplasm (Fig. 1G). Based mostly on actinomycin D expression, circNUP50 was extra steady than linear NUP50 (Fig. 1H). Platinum-resistant OC tissues (Fig. 1I) and cells (Fig. 1J) overexpressed circNUP50.
CircNUP50 was upregulated in ovarian most cancers (OC) platinum-resistant tissues/cells. A Heatmap of round RNA (circRNA) sequencing of platinum-resistant and platinum-sensitive OC tissues. B Scatterplot confirmed a whole lot of circRNA expression variations in platinum-resistant and -sensitive OC tissue. C Kyoto Encyclopedia of Genes and Genomes (KEGG) evaluation primarily based on circRNA-seq knowledge. D The round construction of circNUP50 was confirmed by amplification with divergent primers, adopted by Sanger sequencing, which confirmed that circNUP50 was generated from exon 6 of the NUP50 gene by means of again splicing. E The round construction of circNUP50 and the absence of a poly(A) tail of circNUP50 had been verified utilizing oligo (dT) and random primers. F and H RNase R and actinomycin D expression urged that circNUP50 was extra steady than linear NUP50. G Fluorescence in situ hybridisation experiments demonstrated that circNUP50 was current within the nucleus and cytoplasm of cells. I and J The qRT-PCR outcomes revealed circNUP50 overexpression in OC platinum-resistant tissues and cells
CircNUP50 dysregulation contributes to irregular expression of DDP resistance-associated genes in OC
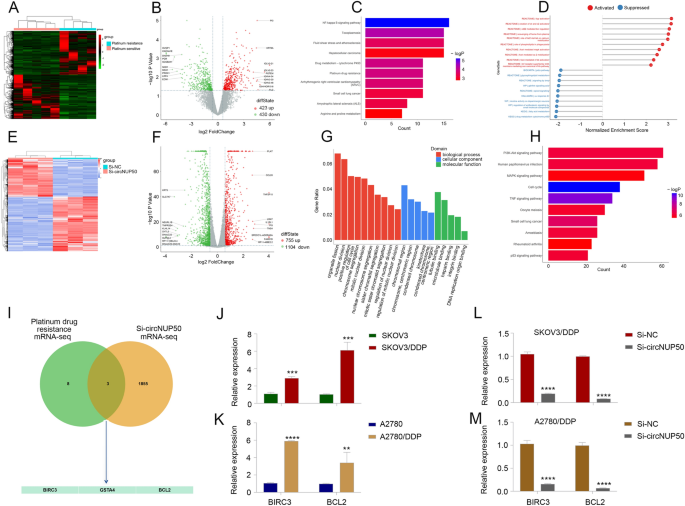
We carried out mRNA-seq on 4 platinum-resistant and 5 PS OC tissues (Fig. 2A and B) and subjected the outcomes to KEGG (Fig. 2C) and gene set enrichment analyses (Fig. 2D) to research the aberrant expression of platinum-resistant and circNUP50-related genes in OC The mRNA-seq knowledge had been carefully related to cytochrome P450 drug metabolism, platinum drug resistance, and MAPK activation regulation.
CircNUP50 dysregulation contributes to irregular expression of platinum resistance-associated genes in OC. A and B Heatmap and scatterplot of mRNA sequencing of platinum-resistant and -sensitive OC tissues. C KEGG evaluation primarily based on mRNA-seq knowledge. D Gene set enrichment evaluation (GSEA) primarily based on mRNA-seq knowledge. E and F Heatmap and scatterplot of mRNA-seq knowledge of si-circNUP50 SKOV3/DDP and si-NC SKOV3/DDP cells. G and H KEGG and GSEA primarily based on si-circNUP50 SKOV3/DDP cells and si-NC SKOV3/DDP cell mRNA-seq knowledge. I Intersection of mRNA-seq knowledge from OC tissues and si-circNUP50-SKOV3/DDP cells J and Ok BIRC3 and BCL2 expression in SKOV3/DDP and A2780/DDP cells. L and M BIRC3 and BCL2 expression in SKOV3/DDP and A2780/DDP cells transfected with si-circNUP50
We additionally carried out mRNA-seq on si-circNUP50 SKOV3/DDP cells (SKOV3/DDP cells transfected with si-circNUP50) and si-NC SKOV3/DDP cells (Fig. 2E and F). GO (Fig. 2G and KEGG (Fig. 2D) analyses revealed that circNUP50 expression was related to optimistic cell cycle regulation in organic processes and the MAPK and p53 signalling pathways. Activating the MAPK pathway reduces the efficacy of platinum medication in OC [22]. We evaluated mRNA-seq knowledge from OC tissues and si-circNUP50-SKOV3/DDP cells and recognized three platinum drug resistance mRNAs, BIRC3, GSTA4, and BCL2, whose perform is mediated by means of circNUP50 (Fig. 2I). Kaplan–Meier evaluation revealed that BIRC3 expression was considerably associated to OS and PPS (Further file 5: Determine S2A, S2D, and S2G), GSTA4 expression was considerably associated to PFS and OS (Further file 5: Determine S2B, S2E, and S2H), and BCL2 expression was considerably associated to PFS (Further file 5: Determine S2C, S2F, and S2I). BIRC3 and BCL2 expression was increased in sufferers immune to platinum-based remedy and was considerably completely different from that in sufferers who did reply to platinum-containing remedy (Further file 5: Fig. S2J–SL). We then explored BIRC3 and BCL2 expression in DDP-resistant OC cells (SKOV3 and SKOV3/DDP cells, A2780 and A2780/DDP cells), and BIRC3 and BCL2 expression was increased in SKOV3/DDP (Fig. 2J) and A2780/DDP cells (Fig. 2Ok). Moreover, BIRC3 and BCL2 expression was lowered in SKOV3/DDP and A2780/DDP cells transfected with si-circNUP50 (Fig. 2L and M). These outcomes recommend that circNUP50 could regulate platinum resistance in OC by means of BIRC3 and BCL2.
CircNUP50 regulates the proliferation potential, cell cycle development, and apoptosis of platinum-resistant OC cells
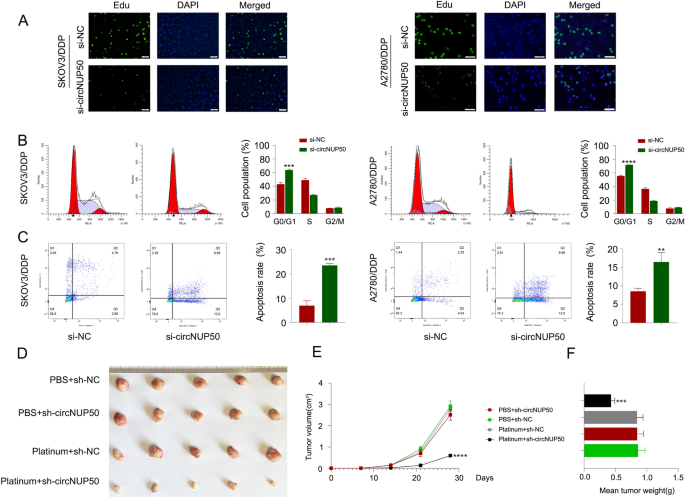
Based mostly on the mRNA-seq outcomes obtained for si-circNUP50 SKOV3/DDP cells, circNUP50 expression was carefully related to optimistic regulation of epithelial cell proliferation, optimistic cell cycle regulation, apoptosis, and drug response (Further file 3: Desk S5). Moreover, we used an EdU assay to look at cell proliferation and circulate cytometry to detect the cell cycle and apoptosis to elucidate the position of circNUP50 in regulating platinum resistance in OC cells. We characterised the phenotypes in SKOV3/DDP and A2780/DDP cells with circNUP50 silencing (si-circNUP50). As si-circNUP50 considerably inhibited circNUP50 expression in platinum-resistant OC cells (SKOV3/DDP and A2780/DDP cells) (Further file 4: Determine S1D), we selected si-circNUP50 for the cell perform experiments. The drug resistance of si-NC SKOV3/DDP, si-NC A2780/DDP, si-circNUP50 SKOV3/DDP, and si-circNUP50 A2780/DDP cells was maintained at a DDP focus of 6 μM. CircNUP50 expression knockdown may scale back the proliferative exercise of SKOV3/DDP and A2780/DDP cells in comparison with that of management cells (Fig. 3A). Cell cycle experiments confirmed that circNUP50 expression knockdown considerably induced cell cycle arrest on the G0/G1 section in SKOV3/DDP and A2780/DDP (Fig. 3B). Apoptosis experiments confirmed that the circNUP50 knockdown elevated the apoptosis price in SKOV3/DDP and A2780/DDP cells (Fig. 3C).
CircNUP50 can regulate the proliferation potential, cell cycle development, and apoptosis of OC platinum-resistant cells. A EdU experiments confirmed that circNUP50 knockdown lowered the proliferative exercise of SKOV3/DDP and A2780/DDP cells. B circNUP50 knockdown led to cell cycle arrest on the G0/G1 section in SKOV3/DDP and A2780/DDP cells. C circNUP50 knockdown elevated the apoptosis price in SKOV3/DDP and A2780/DDP cells. D–F Xenografts fashioned by SKOV3/DDP cells bearing platinum + sh-circNUP50 exhibited a considerably slower progress price (D and E) and decrease tumour weight than xenografts from management teams
Subsequently, we validated the position of circNUP50 in mediating resistance to platinum remedy in OC in vivo. We designed 4 units of managed experiments (PBS + sh-NC, PBS + sh-circNUP50, platinum + sh-NC, and platinum + sh-circNUP50), and tumour xenograft knowledge confirmed no vital distinction in tumour measurement between the 2 teams of mice handled with PBS; nevertheless, downregulating circNUP50 in DDP-resistant cells (platinum + sh-NC group vs. platinum + sh-circNUP50 group) considerably lowered xenograft tumour progress and sensitised the cells to DDP remedy (Fig. 3D). Furthermore, xenograft tumours fashioned by SKOV3/DDP cells carrying sh-circNUP50 displayed considerably slower progress charges (Fig. 3E) and decrease tumour weights (Fig. 3F). These findings recommend that circNUP50 contributes to platinum resistance in OC by selling cell proliferation, affecting the cell cycle, and lowering apoptosis.
CircNUP50 mediates platinum resistance in OC by regulating p53 ubiquitination by binding to UBE2T
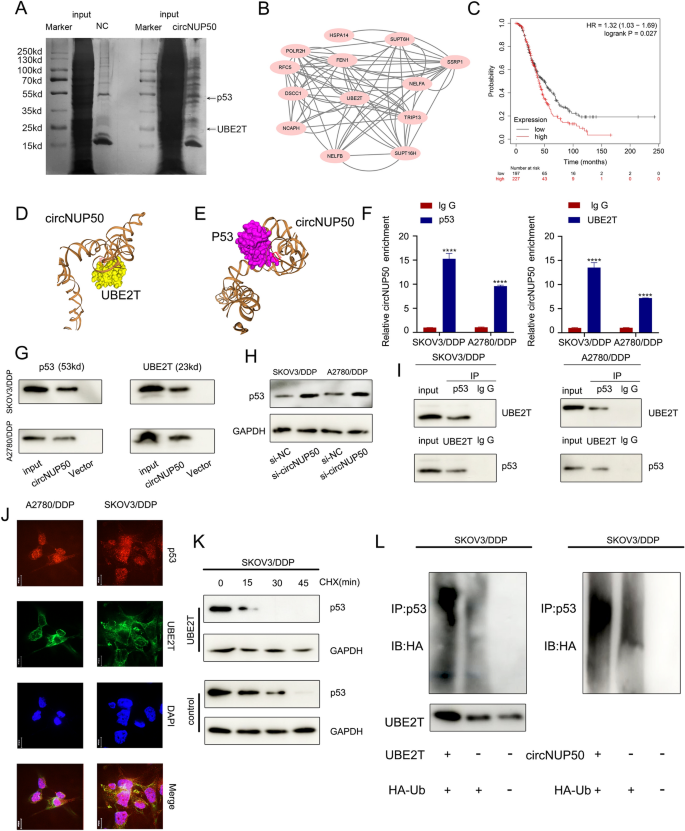
To discover the proteins certain to circNUP50, we carried out silver staining of the RNA pull-down (Fig. 4A), adopted by complete identification of RNA-binding proteins utilizing mass spectrometry (ChIRP-MS) (Further file 6: Determine S3A and S3B). CircNUP50 can bind a whole lot of proteins relative to controls, and PPI (Supplementary Fig. 3C) and GO analyses of those certain proteins (Further file 6: Determine S3D) had been carried out. These analyses confirmed that the binding proteins may mediate ubiquitination modifications within the context of organic processes, mobile elements, and molecular capabilities. GO evaluation of the circRNA-seq, mRNA-seq on si-circNUP50 SKOV3/DDP cells, and mass spectrometry confirmed that circNUP50 regulates ubiquitination and performs a necessary position in platinum resistance of OC. Due to this fact, Ub‐conjugating enzyme E2T (UBE2T), an important ubiquitin-conjugating enzyme among the many binding proteins, attracted our consideration (Fig. 4B). Excessive UBE2T expression was considerably related to poor OS in OC sufferers handled with DDP-containing regimens (Fig. 4C). UBE2T promotes hepatocellular carcinoma cell progress by means of p53 ubiquitination [23]. Apparently, GO and KEGG evaluation of the si-circNUP50 SKOV3/DDP cells mRNA-seq knowledge confirmed that circNUP50 expression regulates p53-related pathways. Due to this fact, we hypothesised that circNUP50 accelerates UBE2T ubiquitination regulation of p53 by means of molecular scaffolding.
CircNUP50 mediates platinum resistance in OC by regulating p53 ubiquitination by binding to UBE2T. A Silver staining of the RNA pull-down confirmed that circNUP50 certain to UBE2T and p53. B Protein–Protein Interplay (PPI) community evaluation primarily based on mass spectrometry knowledge of circNUP50-binding proteins. C UBE2T expression with respect to OS of OC sufferers handled with cisplatin-containing regimens. D and E Molecular simulation outcomes supported that circNUP50 may precisely dock with UBE2T or p53. F and G RNA binding protein immunoprecipitation (RIP) evaluation and circRNA pull-down confirmed that p53 and UBE2T may work together with circNUP50. H Enhanced p53 expression ranges after circNUP50 knockdown. I Co-immunoprecipitation experiments confirmed that p53 and UBE2T may bind to one another. J Co-localisation experiments confirmed that p53 and UBE2T had been co-localised within the cytoplasm of SKOV3/DDP and A2780/DDP cells. Ok Cycloheximide (CHX) chase assays confirmed that the half-life of p53 in SKOV3/DDP cells was lowered when UBE2T was overexpressed. L The ubiquitination experiment confirmed that UBE2T overexpression and circNUP50 in SKOV3/DDP enhanced p53 ubiquitination
We investigated whether or not circNUP50 interacts with UBE2T or p53, and its secondary construction was assembled in Mfold (v. 2.3). circNUP50 can dock completely with UBE2T and p53 (Fig. 4D and E). We carried out RIP evaluation utilizing anti-UBE2T and anti-p53 antibodies to additional characterise the interplay of circNUP50 with UBE2T and p53. p53 and UBE2T interacted with circNUP50 in SKOV3/DDP and A2780/DDP cells (Fig. 4F). TRAP-western blotting confirmed that p53 and UBE2T may bind to circNUP50-MS2 (Fig. 4G). Moreover, western blotting verified that circNUP50 accelerates p53 ubiquitination by means of the molecular scaffold and p53 expression in SKOV3/DDP and A2780/DDP cells elevated after silencing circNUP50 (Fig. 4H).
Subsequent, we verified the ubiquitination regulation of p53 by UBE2T in SKOV3/DDP cells. Co-immunoprecipitation (Co-IP) confirmed that p53 and UBE2T may bind to one another in SKOV3/DDP and A2780/DDP cells (Fig. 4I). Co-localisation experiments confirmed that p53 and UBE2T proteins co-localised within the cytoplasm of SKOV3/DDP and A2780/DDP cells (Fig. 4J). Cycloheximide (CHX) chase assays confirmed that the half-life of p53 in SKOV3/DDP cells was lowered when UBE2T was overexpressed (in comparison with that within the management) (Fig. 4Ok). The ubiquitination experiment confirmed that UBE2T overexpression in SKOV3/DDP enhanced p53 ubiquitination. circNUP50 overexpression additionally enhanced p53 ubiquitination (Fig. 4L). Due to this fact, circNUP50 can bind p53 and UBE2T concurrently and mediate platinum resistance in OC by binding to UBE2T to manage p53 ubiquitination.
CircNUP50 upregulates G3BP1 expression by appearing as a miR-197-3p sponge
circRNA is a molecular sponge that adsorbs miRNAs to manage the expression of downstream genes. We carried out miRNA-seq analyses of platinum-resistant and PS OC tissues to research whether or not miRNAs may be sponged by circNUP50 (Fig. 5A). KEGG evaluation of the info confirmed that miRNAs had been enriched within the platinum resistance-associated PI3K-Akt and AMPK signalling pathways (Fig. 5B). The CSCD (http://gb.whu.edu.cn/CSCD/) and circRNA databases (https://circinteractome.nia.nih.gov/) had been used to foretell miRNA binding to circNUP50 and mixed with the miRNA-seq knowledge from OC tissues; these findings point out that solely miR-197-3p and miR-1296-5p may bind to circNUP50 (Fig. 5C). qRT-PCR confirmed that miR-197-3p expression was increased in OC PS tissues and considerably completely different than in OC platinum-resistant tissues (Fig. 5D). Equally, miR-197-3p expression considerably differed in platinum-resistant and PS cells (Fig. 5E). In contrast, miR-1296-5p expression in platinum-resistant and PS cells was low and fewer vital than that of miR-197-3p (Further file 6: Determine S3E). Due to this fact, we chosen miR-197-3p for the follow-up examine. miR-197-3p can particularly bind circNUP50 primarily based on a circRNA pull-down assay (Fig. 5F). RIP experiments confirmed elevated circNUP50 and miR-197-3p enrichment within the Ago2 group in comparison with that within the IgG group (Fig. 5G). RNA FISH experiments confirmed that circNUP50 and miR-197-3p co-localised within the cytoplasm (Fig. 5H).
CircNUP50 upregulates G3BP1 by appearing as a miR-197-3p sponge. A Heatmap of miRNA sequencing of platinum-resistant and PS OC tissues. B KEGG evaluation primarily based on miRNA-seq knowledge. C Identification of miRNAs that bind to circNUP50. D miR-197-3p expression is considerably completely different in platinum-resistant and PS OC tissues. E miR-197-3p expression is considerably completely different in platinum-resistant and PS cells. F circRNA pull-down assay confirmed that miR-197-3p may particularly bind to circNUP50. G RIP experiments confirmed elevated circNUP50 and miR-197-3p enrichment within the Ago2 group in comparison with that within the IgG group. H RNA FISH experiments confirmed that circNUP50 and miR-197-3p had been co-localised within the cytoplasm. I Identification of particular goal genes of miR-197-3p from three databases. J After combining si-circNUP50 mRNA-seq knowledge, two goal genes of miR-197-3p had been recognized. Ok–M Luciferase exercise was considerably inhibited in cells co-transfected with miR-197-3p mimic and wild-type luciferase reporter genes (circNUP50-WT and G3BP1-WT) in comparison with that within the controls
To elucidate the perform of miR-197-3p, we used an EdU assay to look at the proliferation of platinum-resistant cells; we used circulate cytometry for cell cycle evaluation and apoptosis estimation. The proliferation of SKOV3/DDP (Further file 7: Determine S4A) and A2780/DDP cells (Further file 7: Determine S4B) was lowered when miR-197-3p was overexpressed. Cell cycle assays confirmed that SKOV3/DDP (Further file 7: Determine S4C) and A2780/DDP cells (Further file 7: Determine S4D) had been arrested within the G0/G1 section of the cell cycle when miR-197-3p was overexpressed. Apoptosis experiments revealed that the apoptosis price of SKOV3/DDP (Further file 7: Determine S4E) and A2780/DDP (Further file 7: Determine S4F) cells was elevated after overexpressing miR-197-3p. Moreover, the perform of anti-miR-197-3p was rescued by the lower in circNUP50 ranges.
As well as, we reviewed the miRDB, miRTarBase, and TargetScan databases to seek out particular miR-197-3p goal genes and 30 goal genes had been found (Fig. 5I). G3BP1 and FRMPD3 had been goal genes when the si-circNUP50 mRNA-seq knowledge had been mixed (Fig. 5J). The OS, PFS, and PPS of G3BP1 (Further file 8: Determine S5A–C) and FRMPD3 (Further file 8: Determine S5F–SH) in platinum-containing handled OC had been mapped on the Kaplan‒Meier Plotter on-line web site. G3BP1 expression was increased in sufferers immune to platinum-containing remedy and differed considerably from that of sufferers who responded to platinum-containing remedy (Further file 8: Determine S5D and S5E). In contrast, FRMPD3 expression was not considerably completely different in sufferers who responded or had been immune to platinum-containing remedy (Further file 8: Determine S5I and SJ). Due to this fact, G3BP1 was recognized as a downstream goal gene. Luciferase reporter assay confirmed that miR-197-3p may bind to circNUP50 and G3BP1. When miR-197-3p mimic and wild-type luciferase reporter genes (circNUP50-WT and G3BP1-WT) had been co-transfected into cells, luciferase exercise considerably decreased (in comparison with that within the controls) (Fig. 5Ok–M). qRT-PCR revealed a major lower in G3BP1 expression upon overexpressing the miR-197-3p mimic (Further file 8: Determine S5K). In distinction to sh-circNUP50 transfection, anti-miR-197-3p transfection in SKOV3/DDP cells elevated G3BP1 expression (Further file 8: Determine S5L). These experiments demonstrated that circNUP50 may act as a miR-197-3p sponge to upregulate G3BP1 to mediate platinum resistance in OC.
G3BP1 mediates p53 ubiquitination to manage platinum resistance in OC
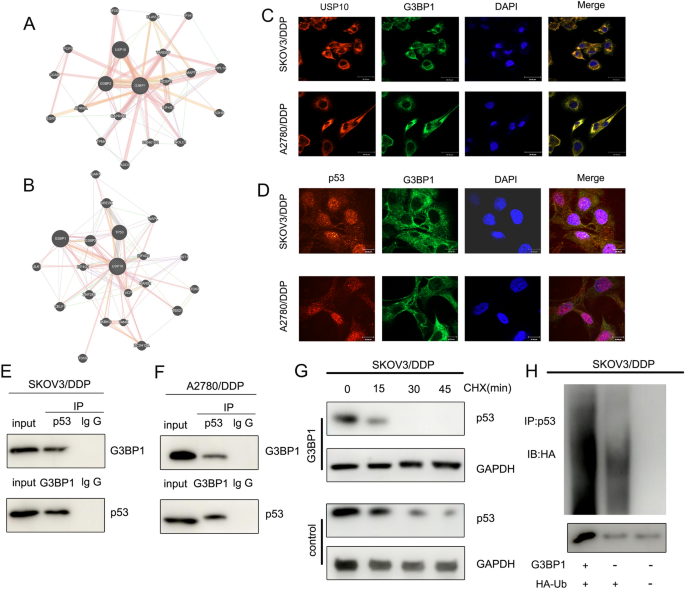
We carried out PPI evaluation and located that G3BP1 interacted with USP10 (Fig. 6A), and G3BP1 was concerned within the ubiquitination of p53 by USP10 [24]. Subsequent PPI evaluation verified that G3BP1 interacts with USP10 and TP53 (Fig. 6B). RNA FISH confirmed that G3BP1 co-localised with USP10 and p53 within the cytoplasm (Fig. 6C and D). Co-IP demonstrated that p53 and G3BP1 had been certain to one another in SKOV3/DDP and A2780/DDP cells (Fig. 6E and F). CHX chase assays confirmed that the half-life of p53 in SKOV3/DDP cells was lowered when G3BP1 was overexpressed in comparison with that within the management group (Fig. 6G). Ubiquitination assays confirmed that G3BP1 overexpression enhanced p53 ubiquitination in SKOV3/DDP cells (Fig. 6H). These experiments recommend that G3BP1 can mediate p53 ubiquitination to manage platinum resistance in OC.
G3BP1 mediates p53 ubiquitination to manage platinum resistance in OC. A PPI evaluation revealed that G3BP1 interacts with USP10. B PPI evaluation outcomes confirmed that USP10 interacted with G3BP1 and p53. C and D RNA FISH confirmed that G3BP1 co-localised with USP10 and p53 within the cytoplasm. E and F Co-IP demonstrated that p53 protein and G3BP1 certain to one another in SKOV3/DDP and A2780/DDP cells. G CHX chase assays confirmed that the half-life of p53 in SKOV3/DDP cells was lowered when G3BP1 was overexpressed in comparison with that within the management group. H Ubiquitination assay confirmed that the G3BP1 overexpression enhanced the ubiquitinated p53 expression in SKOV3/DDP cells
Synergistic codelivery of si-circNUP50 and platinum utilizing nanosystems to beat platinum resistance in an OC tumour mannequin
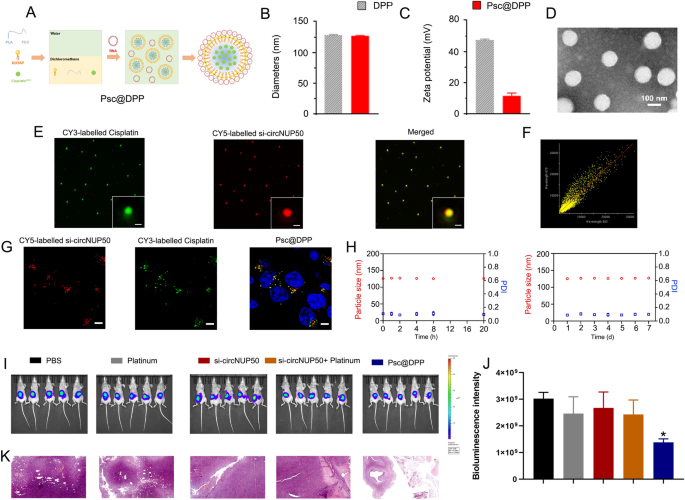
A platinum and si-circNUP50 co-delivery nanosystem (Psc@DPP) had been ready to deal with platinum-resistant OC in an orthotopic animal mannequin (Fig. 7A). Psc@DPP contains polyethylene glycol monomethyl ether-polylactic acid (mPEG-PLA) and DOTAP. As platinum chemotherapeutic medication are hydrophobic, they are often loaded inside the hydrophobic block PLA of Psc@DPP. On account of its optimistic cost, DOTAP can load si-circNUP50 onto Psc@DPP by means of electrostatic adsorption. The particle measurement of Psc@DPP is 150 nm, and the zeta potential is + 15 mV (Fig. 7B and C). Transmission electron microscopy picture of Psc@DPP is introduced in Fig. 7D. To confirm the co-loading of platinum and si-circNUP50 on Psc@DPP, Cy3-labelled platinum and Cy5-labelled si-circNUP50 had been loaded onto Psc@DPP. The fluorescence alerts of platinumCy3 and si-circNUP50Cy5 on Psc@DPP had been photographed with three-dimensional structured illumination microscopy. The fluorescence alerts of platinumCy3 and si-circNUP50Cy5 on Psc@DPP had been statistically analysed utilizing Pearson’s correlation take a look at (Fig. 7E). The Pearson’s correlation coefficient of platinumCy3 and si-circNUP50Cy5 on Psc@DPP was 0.86 (Fig. 7F). After co-incubating Psc@DPP with ovarian tumour cells for 4 h, Psc@DPPs delivered platinumCy3 and si-circNUP50Cy5 into ovarian tumour cells (Fig. 7G). The serum stability of Psc@DPP inside 24 h and the storage stability of the nanoparticles inside 7 days was good (Fig. 7H).
si-circNUP50 and platinum co-delivery utilizing nanosystems to beat platinum resistance in an OC tumour mannequin. A Construction of the Psc@DPP co-delivery nanosystem. B and C The particle measurement of Psc@DPP is 150 nm, and the zeta potential of Psc@DPP is + 15 mV. D The transmission electron microscopy picture of Psc@DPP. E Fluorescence alerts equivalent to platinumCy3 and si-circNUP50Cy5 on Psc@DPP had been statistically analysed utilizing Pearson’s correlation take a look at. F The Pearson’s correlation coefficient of platinumCy3 and si-circNUP50Cy5 on Psc@DPP was 0.86. G After co-incubating Psc@DPP with ovarian tumour cells for 4 h, Psc@DPPs delivered platinumCy3 and si-circNUP50Cy5 into OC cells. H The serum stability of Psc@DPP inside 24 h and the storage stability of the nanoparticles inside 7 days was good. I and J The fluorescence depth was considerably decrease within the Psc@DPP group in contrast than within the PBS and platinum teams, whereas there was no vital change in fluorescence depth within the platinum + si-circNUP50 group. Ok Haematoxylin and eosin staining of OC tissues revealed decreased tumour space within the Psc@DPP group in comparison with that within the different teams
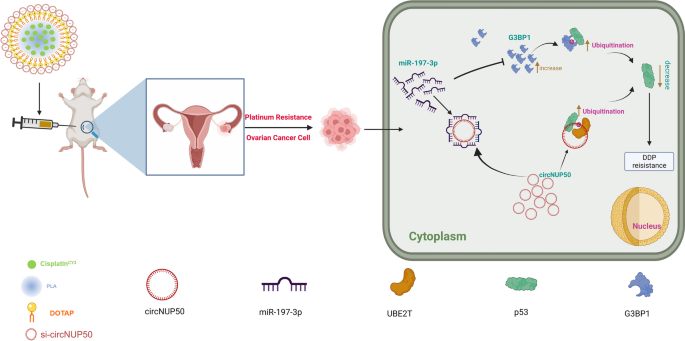
We constructed an OC platinum-resistant orthotopic animal mannequin to discover the potential anti-tumour results of Psc@DPP in vivo. We noticed that the fluorescence depth was considerably decrease within the Psc@DPP group than within the PBS and platinum teams. In contrast, no vital change within the fluorescence depth within the platinum + si-circNUP50 group was noticed (Fig. 7I and J). HE staining of OC tissues confirmed decreased tumour space within the Psc@DPP group in comparison with that within the different teams (Fig. 7Ok). The protection, biocompatibility, and focusing on properties of the Psc@DPP nanosystem had been evaluated. Psc@DPP had security profile (Further file 9: Determine S6A) and brought on no harm to main organs, together with the guts, liver, spleen, lungs, and kidneys (Further file 9: Determine S6B), with higher focusing on properties inside tumour tissues (Further file 9: Determine S6C). As well as, the potential anti-tumour results of Psc@DPP had been explored on the mobile degree in vitro. Within the Psc@DPP experimental group, the proliferation of SKOV3/DDP and A2780/DDP cells (Further file 10: Determine S7A) was attenuated, SKOV3/DDP and A2780/DDP cells (Further file 10: Determine S7B, p < 0.05) had been arrested within the G0/G1 section of the cell cycle, and the apoptosis charges of SKOV3/DDP and A2780/DDP cells had been elevated (Further file 10: Determine S7C, p < 0.05). The outcomes recommend that Psc@DPP successfully overcame platinum resistance in an OC tumour mannequin and presents a novel method for treating platinum-resistant OC utilizing si-circNUP50 (Fig. 8).